Submitted by Administrator on Tue, 01/09/2020 - 09:40
A recent study, led by Dr Aleksej Popel and directed by Professor Ian Farnan, both at the Department of Earth Sciences and Cambridge Nuclear Energy Centre, has observed the surface breakdown of uranium dioxide, the primary component of nuclear fuel, shedding light on the mechanism and rate of waste leaching.
Nuclear power, which currently generates 10 % of global electricity, is likely to become a key player in the green energy transition. However, nuclear energy, although low carbon and reliable, was excluded from the EU green transition fund earlier this year over concerns it may cause harm to other environmental objectives listed under the EU criterion (or taxonomy) for sustainable investment. The inclusion of nuclear in investment plans, in the EU and more widely - where similar green bonds exist, is dependent on safe nuclear waste disposal.
Direct disposal, where fuel waste is sealed in iron canisters and buried hundreds of metres below ground in granite or clay, is now seen as the most viable option. But this isn’t a quick solution – the waste has to be deemed safe by regulators and policy makers for hundreds of thousands, or even millions of years. For scientists, who have no reference over such long time periods, understanding the safety of storage over millions of years is particularly challenging.
The new work, in collaboration with the Pacific Northwest National Laboratory (PNNL), USA, and EU Joint Research Centre, Karlsruhe, brings us closer to understanding the mechanisms of nuclear waste dissolution, under a range of experimental conditions. The team used state-of-the-art scanning transmission electron microscopy (STEM) to capture images, at a rarely seen atomic scale, of the uranium dioxide as it broke down.
Popel and co-authors engineered a thin film, just a few hundred nanometers thick, of uranium dioxide and recorded surface changes as it dissolved in deionized water under anoxic conditions, replicating the oxygen-depleted conditions at geological storage depths. They observed a series of pits and cracks, measuring only a few nanometres across, on the surface of the film as it dissolved. According to Popel “the pits usually form at weaker points in the uranium dioxide where you have a boundary between grains in the crystals”.
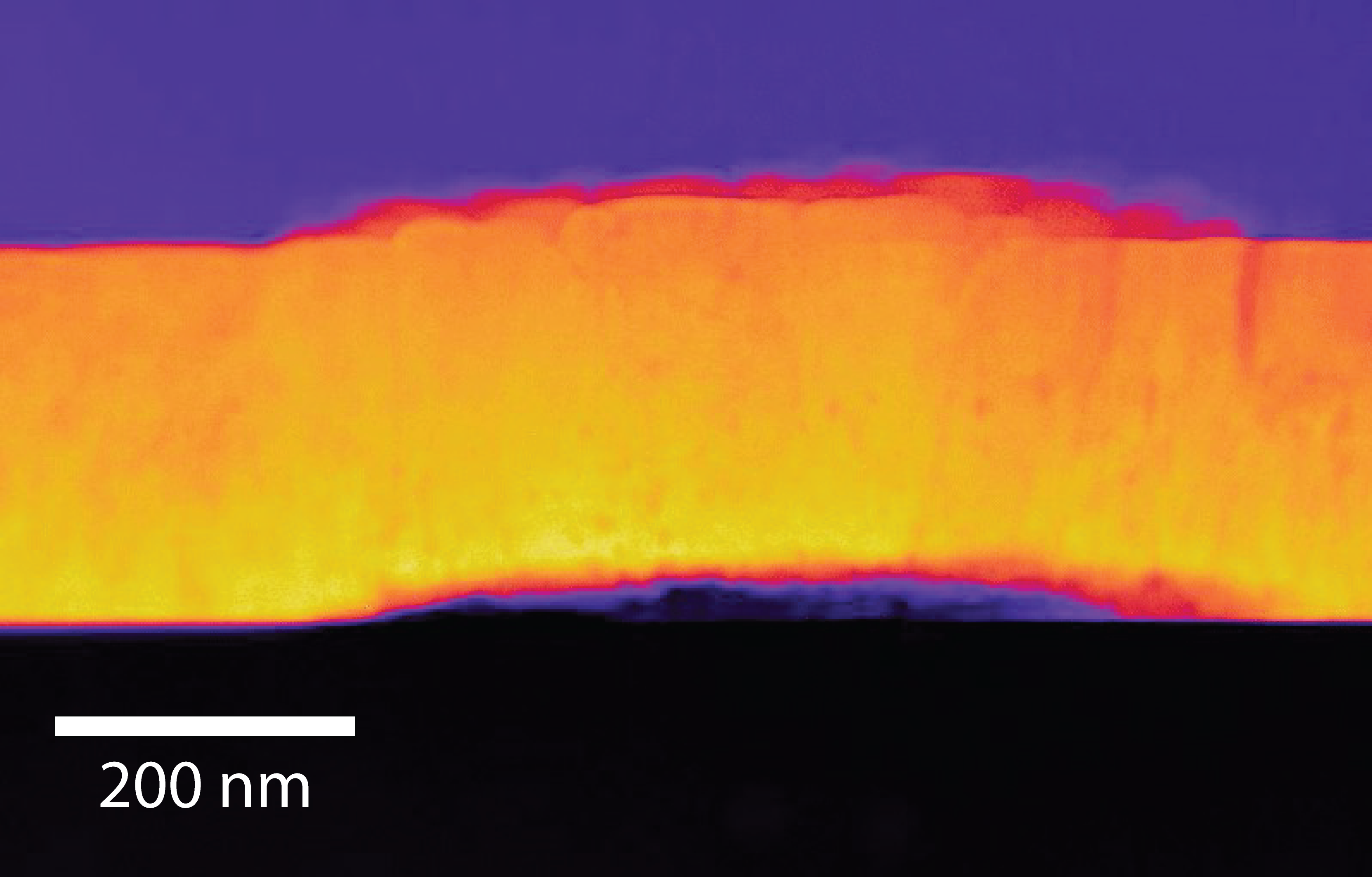
Scanning transmission electron microscopy (STEM) image of a cross section through the uranium dioxide film, showing buckling as it dissolves, image credit: Steven Spurgeon, PNNL.
The study also observed clusters of newly formed crystals that appear to penetrate down through the film. How these secondary crystals, which occur as a by-product of alteration, are formed has remained poorly understood but it is commonly thought that oxidizing conditions are required. “Even with our anoxic experiments, we observed additional oxygen in the atomic layers at the surface of the cracks and pits - it looks as though water alone is enough to supply the oxygen required” said Popel.
It had previously been thought that dissolution would result in the formation of a hydrous amorphous phase at the surface, akin to a gel, “but what we actually see is a crystalline oxidized layer. Using atomic scale chemical imaging we found oxygen embedded throughout the layer, which seems to be acting as a passivation (less-reactive) barrier – explaining why we see a reduction in uranium release with time” said Popel, who cautioned that “this oxidised layer may at some point break down – possibly causing uranium release to increase again”.
“This study is the first to show the dissolution process clearly, an advance only made possible with the nanoscale resolution of scanning transmission electron microscopy and atomic scale chemical imaging, combined with the radiological facilities at the PNNL facility”, said Professor Ian Farnan.
Popel highlights the potential for further work, “we kept the experiment as simple as possible by using a smooth film of uranium dioxide and pure water. It is idealized, but we now hope to add more variables; for instance adding thorium to the film, exposing the sample to radiation damage and using hydrogen peroxide as a proxy for radiolysis products from interactions between water and the radioactive elements present in the spent fuel”.
Popel, A.J., Spurgeon, S.R., Matthews, B., Olszta, M.J., Tan, B.T., Gouder, T., Eloirdi, R., Buck, E.C. and Farnan, I., 2020. An Atomic-Scale Understanding of UO2 Surface Evolution During Anoxic Dissolution. ACS Applied Materials & Interfaces.
